Life Sciences & Health
Other News from the Top Sector Life Sciences & Health
Read about some fantastic developments in the Dutch Life Sciences & Health (LSH) sector last year. Some of these developments will influence the sector in 2018 and beyond. For example, the European Medicine Agency's move to the Netherlands. Check out some of these Dutch pearls below.

EMA to relocate to Amsterdam, the Netherlands ›
Besides strengthening the network of the Life Sciences & Health sector the move of EMA will have a significant economic
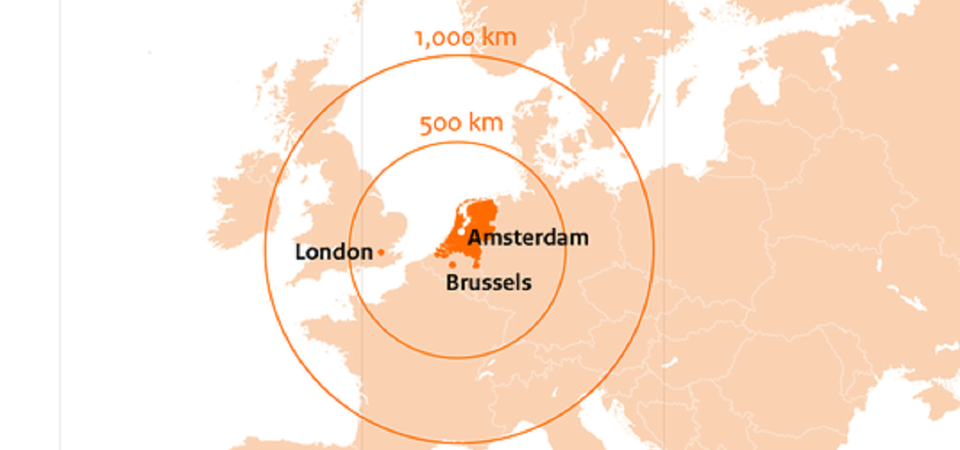
EMA to relocate to Amsterdam, the Netherlands
The European Medicines Agency (EMA) will relocate to Amsterdam in the Netherlands. The agency is responsible for the approval of medicines on the European market. EMA has to relocate due to the United Kingdom’s decision to withdraw from the EU. The Agency immediately began working with the Dutch authorities to prepare for the move and take up its operations in Amsterdam on 30 March 2019 at the latest.
Statement by Wouter Bos, who served as ambassador for Amsterdam’s EMA bid: "Amsterdam scored highly on its proposed premises, easy access, and everything the city has to offer the EMA’s staff. That means Amsterdam is also the right choice for the 500 million Europeans who benefit from the Agency’s work."
Amsterdam was one of 19 offers to host EMA submitted by the Member States at the end of July 2017. Besides strengthening the network of the Life Sciences & Health sector the move of EMA will have a significant economic impact with more than 2,250 fulltime jobs, €150 million direct expenditure and many incoming visitors.
“Amsterdam ticks many of our boxes,” said EMA Executive Director Guido Rasi. “It offers excellent connectivity and a building that can be shaped according to our needs. I am very grateful that the Member States took into account our requirements for business continuity and gave priority to the protection of public and animal health.”
EMA and the Netherlands have agreed a joint governance structure to steer and oversee the relocation project. Because of its important role to safeguard public and animal health in the EU, EMA is committed to giving stakeholders and the public full visibility of the relocation project. The Agency plans to publish a monitoring chart on its website that allows to track the progress made.
Response from the Minister for Healthcare, Welfare and Sport, Bruno Bruins: "This is good news for all patients across Europe. In Amsterdam the EMA will be able to continue its important work without interruption after Brexit. The Agency can continue to grant access to new, innovative medicines without delay and will still be able to respond quickly and effectively in the event of problems with a given drug. Both aspects of the EMA’s role are crucial for all patients in Europe, and we are delighted that the EU made its choice on that basis. I want to thank everyone who contributed to this fine achievement. Now, the real work begins. Amsterdam and the Netherlands as a whole are fully committed to ensuring the EMA’s relocation runs smoothly. We are ready to get started and we will make sure that the EMA’s important work is not disrupted."
Hans Schikan: “The arrival of EMA to our capital will provide a further boost to an already blooming sector, where working together is high on the agenda.”
Source: EMA and Topsectoren

Patients with Parkinson’s disease benefit from specialised physiotherapy ›
ParkinsonNet approach: fewer complications, lower costs
Patients with Parkinson’s disease benefit from specialised physiotherapy
ParkinsonNet approach: fewer complications, lower costs
Specialised ParkinsonNet physiotherapy leads to better care at lower costs. This is the conclusion of large-scale research conducted by the CZ healthcare insurance company and Radboudumc, who studied the medical claims declarations of over four thousand people with Parkinson’s disease for a period of three years. The specialised physiotherapists needed fewer treatment sessions, and the patients sustained fewer complications. The healthcare costs were also significantly lower. The results of this new research will be published in The Lancet Neurology on 13 December, 2017.
There is increasing evidence that patients with Parkinson’s disease benefit from allied health interventions, such as physiotherapy or occupational therapy. Parkinson-specific expertise is needed for optimal treatment of people with Parkinson’s disease. Professionals who have been trained as part of the Dutch national ParkinsonNet have this expertise. Previous scientific research showed that the ParkinsonNet approach results in better care at lower costs. However, little was known about the added value of this approach in the long-term, and in day-to-day practice. Therefore, the healthcare insurer CZ initiated an analysis of their medical claims database including 4,381 patients who had data available for a period of 3 years.
These results show that patients who had been treated by a specialised physiotherapist sustained significantly fewer complications such as bone fractures, and were also less likely to be admitted to a hospital. There was also a tendency towards a lower mortality rate among those who had received the specialised treatment. The costs of this specialised physiotherapy were also significantly lower. On average, patients claimed almost € 400 less per year for specialised treatment than for regular treatment, and the total annual healthcare costs (including the medical specialist care) were on average € 530 lower per patient, because hospital admissions were avoided. In the Netherlands, this represents an estimated annual cost saving of over € 11 million.
Researcher Jan Ypinga from CZ is pleased with the results: “This is the first time that research into this subject has been carried out among such a large, representative patient group, and over several years.
We now have insight into the impact of specialised treatment in everyday clinical practice. I’m glad that patients experienced fewer complications, such as a bone fracture after a fall, if they received specialised physiotherapy. This is really good news for people with Parkinson’s disease. And it enables patients and professionals to make a conscious choice for high-quality care.’
Researcher Jan Ypinga from CZ: "This is really good news for people with Parkinson’s disease. And it enables patients and professionals to make a conscious choice for high-quality care."
Professor Bas Bloem from the Radboudumc mainly emphasises the importance of specialised care for a complex disorder such as Parkinson’s disease: “This analysis of medical claims data sheds new light on the added value of ParkinsonNet in everyday clinical practice. These new findings make it very clear that people with Parkinson’s disease deserve to be treated by an expert with a deep understanding of this complex condition, and who is fully up-to-date on all the latest insights and treatment options.”
Source: Radboudumc

More highlights of 2017
It was a great year for Parkinson’s research & development and ParkinsonNET in 2017, read some of the highlights here:
Making a difference in global healthcare by the Product Development Partnerships ›
Investing in global health
Making a difference in global healthcare by the Product Development Partnerships
Investing in global health
There has been significant progress made over the last decade in research and development (R&D) and innovations in global health. But more than 10 million people worldwide are still dying each year from infectious diseases such as tuberculosis, HIV/AIDS, malaria and other poverty-related diseases and conditions. Many countries have large-scale shortages of effective, accessible and affordable medicines, vaccines, diagnostics and other healthcare products.
Only a small proportion of global health research focuses on conditions that account for a large proportion of the global disease burden. Therefore, the Ministry of Foreign Affairs and the Netherlands Enterprise Agency (RVO) started the Product Development Partnerships (PDP ) III Fund in 2015. This PDP fund contribute to innovation of healthcare products and technologies, specifically aimed at diseases and conditions related to poverty and sexual and reproductive health and rights.
The following PDPs are funded by the ministry of Foreign Affairs:
- Drugs fr Neglected Diseases initiative (DNDi)
- Fundation for Innovative New Diagnostics (FIND)
- Medicines fr Malaria Venture (MMV)
- The Glbal Alliance for TB Drug Development (TB Alliance)
- Internatinal AIDS Vaccine Initiative (IAVI)
- Internatinal Partnership for Microbicides (IPM)
These PDPs can affect a large group of people suffering from terrible diseases. Combating diseases and providing shared solutions in the field of care, cure and prevention is necessary when we are facing the global challenges ‘Health & Care’. Hence it is great to see the fruitful developments within the PDPs/ Some highlights of 2017 as a result of the PDP funds are:
- IPM announced the start of the first clinical trial of IPM’s dapivirine vaginal rings for three-months to prevent HIV in women.
- New MMV-supported products seem effective in patients suffering severe malaria.
- DNDi’s phase II/III studies show high efficacy and safety of the first oral treatment for sleeping sickness.
- FIND and partners completed a first significant trial showing how to reduce antibiotic overuse in developing countries.
- IAVI and partners acquired new data on how some people generate powerful HIV-blocking antibodies, an important insight for the development of a potential AIDS vaccine.
- TB Alliance introduced new TB cures for children and will ensure they are widely available. 700,000 treatment courses have already been ordered.
Find more information on the website.

Centres of expertise collaborate on co-creation with patients ›
Four Centres of Expertise focused on health and wellbeing, work together to enhance co-creation with patients
Centres of expertise collaborate on co-creation with patients
Four Centres of Expertise focused on health and wellbeing, work together to enhance co-creation with patients. Knowledge about methods of co-creation in healthcare from different networks has been brought together in the publication Shared Insights on Co-Creation in Healthcare and was presented to Erik Gerritsen, secretary-general at the Ministry of Health, Welfare and Sport.
Of the 20 plus centres of expertise financed by the Ministry of Education, Culture and Science four are focused on healthcare. EIZT in Limburg is the centre of Innovative Care and Technology, the Centre of Expertise Healthy Ageing in Groningen works on knowledge and solutions for healthy ageing, Generade in Leiden focuses on genomics and U CREATE, Centre of Expertise Future Health Design in Utrecht connects knowledge, methodologies and technologies from the creative industry to the demand for renewal healthcare. The centres possess a lot of knowledge about collaborating with citizens and patients to develop appropriate solutions for the future. This knowledge has been brought together in the publication Shared Insights on Co-Creation in Healthcare. The publication was handed to Erik Gerritsen at the Ministry.
Erik Gerritsen: “I appreciate the addition to the ecosystem of healthcare innovations by the four centres and their networks.”
Co-creating with the patient
It is evident for all four centres that citizens and patients are the most important party to create future care with, but that they are also the most complex group to incorporate in the process. At which stage should a patient be incorporated in the design process, for instance? At the very beginning, or only after there is a working prototype? And what are the right questions to present to them? Are the people in your panel a correct representation of the target audience of your innovation?
Which method is most suited to gather insights from patients? A panel? Or the Participation Ladder? Design Thinking maybe? Or a more traditional marketing method with persona? And how can you reach the difficult targets, such as young people, illiterates or non-western migrants?
The centres share the opinion that these types of questions are best answered when different disciplines work together. Healthcare does not have to develop every needed innovation on its own and can use methods from other sectors. On the other hand, creatives such as marketers and designers, learn from healthcare about ethics and meaningful collaborations with vulnerable people.
Collaboration with Health, Welfare and Sport
By presenting the publication, the four centres offered their services and network to the Ministry of Health, Welfare and Sport. Together they represent over a hundred lectors in the field of health and wellbeing, technology and the creative industry. These lectors, their research groups and students can conduct and use their practical research to rapidly establish and validate innovations. Moreover, the centres have networks of dozens of companies, healthcare organisations, government bodies and knowledge institutes. And because they work closely together with different organisations in healthcare, access to patients and citizens - whom they are eager to involve in their activities - is relatively easy to organize.
The full publication can be ordered on the website of U CREATE

Many new drug approvals in 2017 ›
After disappointing 2016, new drug approvals roared back to life in 2017
Many new drug approvals in 2017
After a disappointing year in 2016, with only 22 new medicines receiving approval from the FDA in the United States, the FDA wasted no time in 2017, signing off on 12 drugs in the first quarter alone – besting its record for the same period of any year in recent history. In total, 46 new medicines received FDA approval. There was also an increase in Europe, from 91 approved medicines in 2016 to 92 in 2017 (both including generic medicines).
Various media went over the figures for 2017 and came up with analyses and conclusions concerning this strong increase. These showed, amongst other things, that the increase can in part be explained by the rising number of medicines that receive the ‘breakthrough status’. Due to this status they are subjected to less stringent requirements before approval. Another striking conclusion is that although a lot of new medicines were approved, the number with new modes of action was comparatively low. Finally it became clear that cell and gene therapies like CAR-T are on the rise and gaining a foothold.
Read for an English analyses and further information this article of Fierce Biotech.
Or find more information on Dutch analyses in the Financieele Dagblad

Open innovation improving drug safety evaluation ›
A new model for sharing data and knowledge for the purpose of improving the toxicological evaluation of drugs

Open innovation improving drug safety evaluation
The Hospital del Mar Medical Research Institute (IMIM) and the Pompeu Fabra University (UPF) have just published a comment in the prestigious journal Nature Reviews Drug Discovery where they explain the excellent results from eTOX, a project that has facilitated a new model for collaboration among pharmaceutical companies, as well as between these and academia, where data and knowledge are shared for the purposes of improving the toxicological evaluation of drugs.
Apart from the results obtained, which are extremely valuable, the project is a model of Open Innovation, where various public and private stakeholders join forces and actively collaborate. In addition, it has confirmed the enormous value of the data obtained in the regulatory studies conducted by the pharmaceutical industry, and has verified the fact that exploiting these requires a significant effort in terms of extraction, standardisation, and integration.
"The eTOX project has given us a glimpse into the potential of this data for constructing predictive models capable of foreseeing effects in vivo. However, it is necessary to incorporate toxicodynamic effects and analyse the extent to which the experimental data and predictions can be extrapolated to humans. To this end, we have just launched the new project eTRANSAFE", explains Ferran Sanz, the project coordinator and Director of the IMIM and UPF Biomedical Informatics programme (GRIB).
"One of the 'intangible' results of the project is the construction of a common language between partners with different professional profiles. Today, the computing experts know much more about toxicology than they did at the beginning of the project and we have a much deeper understanding of the problems and needs that exist in this area. The toxicologists have also learned a great deal and now have a better appreciation of the possibilities offered them by advanced computer and information technologies", adds Manuel Pastor, head of the Pharmacoinformatics group at the GRIB.
An added value of the eTOX project is the development of technology that allows research results to be transferred to companies and sustainable results generated that can continue to be used beyond the end of the project.
Reference article
“Legacy data sharing to improve drug safety assessment: the eTOX project” Ferran Sanz, François Pognan, Thomas Steger-Hartmann, Carlos Díaz and eTOX (including Manuel Pastor). Nature Reviews Drug Discovery, 2017.
Source: IMIM
